Synthetic zeolites, CAS No. 1318-02-1, comprise a group of synthetic, crystalline aluminosilicates made out of silicon dioxide (SiO2) and aluminum oxide (Al2O3) in various compositions, together with metal oxides. They are normally produced by mixing the starting materials (an aluminate, a silicate, sodium hydroxide and optionally further metal oxides and templates) in an aqueous solution and crystallized at elevated temperature to form a slurry of crystals. These crystals are separated by filtration, washed with water and dried. Through partial ion exchange (surface modification) the initial product may be modified.
Synthetic zeolites were produced and marketed for many decades without significant changes in its physical properties. The range of synthetic zeolites has become wider, as over time further metal oxides were used for synthesis. Synthetic zeolites are used as detergent additives in the form of dried white powders, in the field of adsorption and catalysis as dehydrated white powders or as formed bodies. In addition, synthetic zeolites are also approved as food contact additives, and as food additives, although with restrictions.
As zeolites are porous substances, containing defined pores (channels) up to 1 nm, they are affected by the evolving discussion on nanomaterials. The purpose of this statement is to provide information that industrially produced zeolites are not considered to be nanomaterials. The statements given are mainly based on the “EU Commission Recommendation 2011/696” (published on 18th October 2011) intended to be applied as an overarching framework with regard to other EU regulatory definitions. In this recommendation nanomaterials are defined as “a natural, incidental or manufactured material containing particles in an unbound state or as aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more of the external dimensions is in the range 1 nm to 100 nm, or where the specific surface area by volume of the material is greater than 60 m2/cm3.”
It must be highlighted that the long standing success of synthetic zeolites is not related to the particle dimensions but to the internal pores (channels), which are typically in the range of 0.3 – 1.0 nm (10 Angstrom) in size and thus in the sub-nanometer range. These pores are responsible for the extraordinary performance of zeolites and not the crystal size of the zeolites. Decreasing the size of the crystals does not change the chemical properties and the specific surface of the zeolites. The pores are structure inherent and independent of the crystal size. They are of the size of small molecules. As the porous structure of the zeolites is independent on the crystal size, the surface area using the BET method cannot be used to describe whether zeolites are nanomaterials or not. BET measurement may provide false results based on the pore size. Further, the pores are in the sub-nanometer range and thus not covered by the above mentioned definition.
A few commercially available zeolites were analyzed in different ways to show that the definition of nanomaterials does not apply to zeolites.
X-Ray powder diffraction analysis first of all shows that zeolites are highly crystalline substances. Industrial zeolites are polycrystalline materials with a crystal size of 1 – 10 µm. The crystal lattice contains defective sites, leading to intergrown crystals that are fixed together by covalent chemical bonds.
Depending on the pore structure and the ion exchange status of the zeolite, a BET surface area of a few m2/cm3 up to a few hundred m2/cm3 can be measured. As mentioned above, the BET method is not an adequate tool to determine whether zeolites are nanomaterials or not. Using the t-plot method, all commercially produced zeolites have an outer surface area in the range of 20 m2/cm3. The outer surface area is dependent on the crystal size. A small outer surface area is a strong indication, that the zeolite crystal size is not in the nanometer range.
Laser diffraction is a widely used tool in industry to determine the particle size of a crystal. The more modern instruments use a dual wavelength laser system and can determine the particle sizes between 20 nm and 2 mm. The results obtained with laser diffraction were compared with results obtained by other, more labor intensive methods, such as scanning electron microscopy (SEM) and aerosol analysis. All of the studied zeolites have hardly any particles below 1 µm and thus do not fall under the definition of nanomaterials. The coincidence of the different measuring methods is sufficiently good.
Further, the zeolites were treated with various dispersing systems to disintegrate potential agglomerates. Ultrasonic treatment for different times, rotating brush generators and sedimentation shafts were used at different energy levels. All these treatments did not lead to a disintegration of the zeolite crystals.
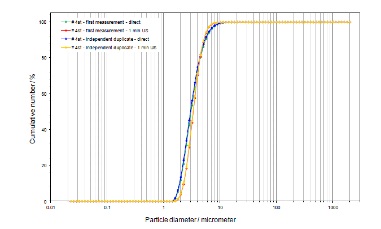
The graphic representation shows the result of a laser diffraction measurement made with a commercial LTA zeolite. The blue and the green curve show the crystal number size distribution before treating with a dispersing system, the red and the yellow curve are produced after the treatment of the zeolite with an ultrasonic system. The blue and the green curve are nearly identical, indicating that the measurement is very well reproducible. The ultrasound treatment leads to minor shifts of the curves only, indicating that the zeolite crystals are not agglomerated. The presence of nano-particles was not evidenced even following ultrasonic treatment. The particle size distributions evidenced were almost identical to those measured upon analysis of the SEM images. Similar results were obtained for the FAU zeolite structure type.
In conclusion, commercially produced synthetic zeolites are not nanomaterials. They do not fulfill the definition given in “Recommendation 2011/696” of the EU Commission. The zeolite crystals are larger than 100 nm and do not disintegrate upon application of dispersing systems (ultrasonic waves or shear forces).
Using modified synthesis procedures, it is possible to manufacture zeolite crystals smaller than 100 nm. These zeolite crystals are stable in diluted suspensionsonly These zeolites are a niche product and are neither covered by this statement nor by the existing REACH registrations of the Synthetic Zeolite Consortium and its member companies.